5039
0
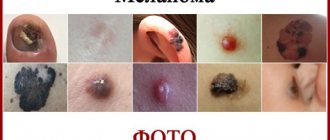
Skin melanoma is a serious cancer that manifests itself in the form of malignant neoplasms. It develops thanks to pigment cells - melanocytes, which begin to divide rapidly and chaotically. The main location of the tumor is the skin; much less often, formations appear on the mucous membranes and nails. In the treatment of melanoma, a number of modern medical procedures and techniques are used; they can eliminate lesions, increase the chances of remission and life prognosis. But what is the survival prognosis when this disease is diagnosed and who is at risk? The answers to these questions are below.
Types of melanoma
There are three types of melanoma:
— The superficially widespread type of the disease is considered to be one in which a pigment spot of a rather small size is formed on the skin, only about 5 mm. Its color is dark brown or black. This spot is impossible to notice by touch, since there is no elevation above the skin.
— The nodular type can be classified as a disease when the formation resembles a node, polyp or fungus. It has a blue-red, sometimes black, color.
— Lentigo maligna is considered when the disease has a long period of development. A transformation occurs, and a pigment is formed that has an irregular shape with scalloped outlines.
So, if a person has a birthmark, growth or simple mole, the outlines of which have begun to change, it is necessary to immediately consult a doctor. It is important to understand that the sooner the disease is detected, the earlier its stage, the better the life prognosis and the simpler the treatment if diagnosed with skin melanoma.
Let's consider each stage of the disease separately.
What is melanoma and its features
Skin melanoma is a pathology that represents specific neoplasms formed from dermal cells that produce melanin. The prognosis depends on the stage. In most cases, it is unfavorable, due to the rapid progression of the disease - the spread of metastases.
The etiology of occurrence is due to the transformation of the genome of cells, as a result of which they acquire pathological properties. The negative impact is revealed due to the systematic exposure of the skin to ultraviolet rays (visiting a solarium).
Genetic predisposition is noted as a provoking factor. If it is present, eye melanoma develops more often, regardless of the person’s age.
The most common location is the skin. Less common:
- mucous membrane of the eyes;
- oral cavity;
- anus;
- external genitalia in women.
Melanoma is a tumor that is a type of malignant cancer.
It ranks 6th among the most dangerous diseases in men, 2nd place in women (cervical cancer is in first place). Pathology can develop independently. In most clinical pictures it looks like birthmarks that do not cause concern for a long time. Early diagnosis contributes to a favorable prognosis.
Within one year, it metastasizes to the lymph nodes, then to the bone tissue, liver, lungs and brain. This reduces the overall survival rate to 10%.
How does the treatment work?
Exactly the same as in the first stage, the skin is excised, but the healthy area is covered more. A biopsy is performed. In addition, drug therapy with Interferon is indicated.
In some cases, melanoma in the second stage is removed using radio waves. This allows you to avoid complications after surgery. In this case, the tissue is cut without contact by a high-frequency wave, which provokes evaporation of the desired area of skin.
Cells that have evaporated in this way form steam at a very low temperature, which allows coagulation of nearby vessels. This method is used in both the third and fourth stages. This is how skin melanoma is treated at the second stage.
What does relapse of melanoma look like?
Recurrent neoplasm differs from primary melanoma not so much in appearance as in the total volume of the lesion. Often secondary formations are very similar to the previously removed maternal one, only there are many more of them, they can be larger or smaller.
In domestic oncology, there are four types of melanoma relapses according to Wagner’s classification:
- local - the tumor is located exactly in the area of the primary operation;
- transit metastases or satellites - next to the scar, but no closer than 2 centimeters, can be localized not only in the skin, but also in the subcutaneous tissue;
- regional metastases - damage by a malignant process to the lymph nodes near the operation area;
- distant metastases - secondary melanoma tumors in other organs and tissues.
The patient can have relapses of any type and in any set, all at once, which is not surprising for such an aggressive tumor.
Third stage
At the third stage, the boundaries of melanoma are violated; it invades adjacent tissues. The lymph nodes that are located near the tumor are affected. This can be detected by biopsy.
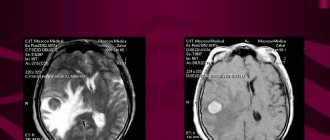
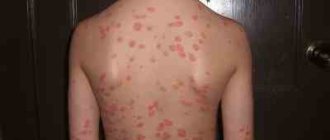
Melanoma of the skin is very dangerous. Life prognosis (stage 4; see photo below) will be discussed later.
What symptoms require immediate medical attention:
- bleeding from a mole or pigmented area;
- the mole begins to grow asymmetrically;
- the skin darkens without tanning;
- the diameter of a mole or birthmark has increased to 6 mm;
- The mole has uneven edges.
Treatment in the case of the third stage will be surgical removal of the tumor from the surface of the skin, and it will also be necessary to remove the lymph nodes that are located next to the tumor.
In this case, drug treatment is carried out using Interferon, Altevira, Ipilimumab.
Once the operation has already been performed, the treatment does not stop. The doctor must keep the patient under observation for some time. At the same time, the necessary tests are taken from the patient. This is different from the treatment of the third stage when diagnosed with skin melanoma. Life forecasts (photo below) are no longer as optimistic as with the other two. 50% live 5 years after surgery, 18% live 10 years.
Prognosis for recovery upon diagnosis
The prognosis for life with melanoma depends on the stage and severity of the course, the location of the pathological neoplasm.
The results of patient therapy are assessed in accordance with five-year survival rate, divided by the degree of the oncological process:
- Zero. The patient lives a long time, survival rate is 99.9%.
- First. There is a good effect of anticancer treatment in 85-95% of patients. Provided that the lesion is up to 2 mm without ulceration.
- With the second, the 5-year survival rate is up to 79%. The disease occurs along with tumor mutation up to 4 mm, and ulcerative lesions are observed.
- At the third stage, only 24-70% of patients live for 5 years.
- The terminal or final stage is characterized by a minimal percentage of patient survival. Survival is possible only in 7-10% of cases.
Melanoma is an oncological disease of a malignant nature. Missing time for adequate treatment can cost lives.
With early access to a medical institution and diagnostic measures, it is possible to stop malignancy - reduce the risk of harmful consequences, and accordingly, the chances of a favorable prognosis increase.
Fourth stage
This is the most dangerous stage of the disease. Cancer cells quickly spread to neighboring areas of the body. Metastases are found in internal organs.
Signs may vary, since they are directly dependent on which organ is damaged:
- the occurrence of a constant cough;
- lumps can be felt under the skin;
- headache with varying intensity;
- a person loses weight;
- occurrence of seizures.
How is stage 4 melanoma treated? Unfortunately, the life prognosis is unfavorable. The ability to recover depends on the process of metastasis. Disease at this stage is treated with surgery, immune therapy, radiation therapy and chemotherapy.
Viritotherapy is a new way to treat melanoma. This is the drug Rigvir, which contains a large number of oncolic and oncotropic viruses.
When they enter the body, they begin to fight malignant tumor cells. In addition, the immune system is stimulated, thus protecting the body from destruction due to a disease such as skin melanoma.
Signs and dangers
At the initial stage of the disease, there are no differences between a pigmentless nevus (mole) and malignant neoplasms. Melanomas show symptoms in moles and healthy skin.
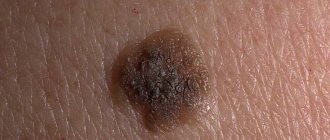
The pathology has a number of characteristic symptoms, according to which the doctor makes an accurate diagnosis. The main symptom is a change in the shape, size and color of an existing mole.
Hairy nevi never become malignant.
Clinical manifestations in the initial phase of development:
- Asymmetricity of the neoplasm.
- Inhomogeneity of color (spot darkens or brightens).
- Increase in diameter over 5 mm.
- Lack of clear edge boundaries.
- Increasing altitude - the flat homeland begins to rise.
- Pathological discharge from the growth.
- Burning, itching, bleeding of moles.
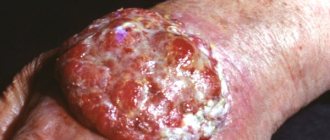
Stage 3 melanoma is characterized by the appearance of pigmentation around the growths, disruption of the integrity of the tumor, constant bleeding and itching. This can be seen in photos of patients on the Internet.
Lack of timely treatment leads to disruption of the functioning of internal organs and systems. Pain syndrome of various localization develops. Metastases affect the liver, brain and bone structure of a person.
Due to the rapid progression of the disease and an increase in the number of cancer cells, intoxication of the body occurs. In combination with disruption of internal organs, this leads to death.
We recommend reading
- Is it possible to cure melanoma: methods, statistics
- Melanosis (melanopathy): causes, symptoms, treatment
- Prognosis for stage 1 skin melanoma: what is life expectancy?
Development of complications after melanoma
The specialist must explain exactly how to carry out rehabilitation after surgical removal of the tumor. The wound surface must be carefully cared for.
If the patient notices the following symptoms, then it is necessary to immediately see the attending physician and undergo a re-examination:
- chills and fever;
- swelling has appeared near the incision, blood and other types of discharge come out of it, there is redness;
— the site where the tumor was removed hurts so much that pain medications do not help;
- a new formation arose in the old place.
Relapse groups
In 1985, the domestic oncologist Anisimov proposed, for the convenience of describing the clinical picture, to divide recurrent tumors into six groups:
- The first - round and few formations, often outside the scar and mainly subcutaneously - in the fatty tissue, often fall under the criteria of “transit” metastasis according to Wagner.
- The second is multiple irregular cutaneous and subcutaneous infiltration accompanying vessels and nerves; it is assumed that the external picture of relapse is formed by tumor cells that have taken root in small vessels.
- The third is nodules directly associated with the operation area that have grown from malignant cells remaining in the skin.
- The fourth is polycyclic multiple formations.
- The fifth is a lot of bulging nodules, often on a stalk, like morel mushrooms.
- The sixth is a combination of all five options.
Classification is rarely used in clinical practice, because the assessment of treatment results is based solely on the size of the nodes, and not on their appearance.
Reviews
With the disease skin melanoma, life prognosis (reviews confirm this) completely depend on the degree of its development and timeliness of treatment.
Patients say that melanoma cannot be cured using traditional methods. This can only help relieve symptoms, but will not get rid of the tumor. The disease will progress, moving into the most severe late stages. Don't waste precious time and risk your own life.
We have reviewed skin melanoma, and the prognosis for life in Russia is also described.
Who is at risk for melanoma?
Skin melanomas grow from ordinary bad tastes that are on the body of any person. They are small benign formations. Although bad tastes themselves are absolutely safe, there are a number of factors that can provoke the development of cancerous processes in them and cause degeneration.
The risk group for developing the disease includes the following categories of people:
- people with pale or light skin, blond or red hair, eyes of blue, gray and green shades;
- people who have over fifty moles on their body;
- people with a genetic predisposition, poor heredity;
- people who have a weak immune system.
The disease can also develop in those who are constantly exposed to sunlight or ultraviolet radiation without protection. Excessive enthusiasm for solariums or uncontrolled sunbathing processes is one of the main factors in the occurrence of pathology.
Algorithm for early detection of skin melanoma in a district clinic
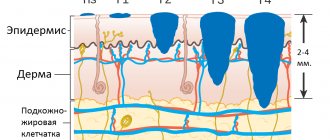
Skin melanoma is a tumor that originates from melanocytes, cells of neuroectodermal origin. Melanocytes are located in the basal layer of the epidermis in a ratio of 1:10 to keratinocytes and synthesize the pigment melanin, which is transmitted through cell processes to keratinocytes to protect the skin from ultraviolet radiation.
The tumor in most cases affects the skin, but can also occur on mucous membranes covered with stratified squamous epithelium, in the retina and iris, and in the soft meninges. Melanoma of the skin is diagnosed visually, since the tumor continues to synthesize the pigment melanin and in most cases is colored.
Despite the visually detectable location and conspicuous dark color of melanoma, every third patient in Russia dies from it. Thus, in 2020, the incidence of melanoma in the country was 7.7 per 100 thousand population, mortality - 2.5, in Moscow in the same year - 9.0 and 3.6, respectively [1]. In the USA, Australia, and New Zealand, the mortality rate is 13–20% [2]. This situation is due to the fact that in the Russian Federation there is insufficient detection of melanoma at an early stage compared to foreign countries.
Thus, in the United States in 2007, there were 59,940 cases of invasive cutaneous melanoma and an additional 48,290 cases of in situ melanoma [2]. In Russia in 2020, 11,057 melanoma patients were registered; stage 1 was detected in 34.6% of cases. How many cases of melanoma in situ have been identified is unknown, because stage 0 melanoma (TisN0M0) in Russia has not yet been recorded.
There are three main forms of cutaneous melanoma: lentigo, superficial spreading and nodular. Some authors identify a fourth form, acrolentiginous, which often occurs in representatives of the Negroid race.
The first two forms, which make up 80% of all melanomas, as well as acrolentiginous, go through two phases of development - the phase of horizontal and vertical growth.
In the horizontal growth phase, melanoma spreads within the epidermis and is not yet capable of producing lymphogenous and hematogenous metastases, since blood and lymphatic vessels are located in the dermis. The five-year survival rate in this phase is 95–98% [2].
In the vertical growth phase, when the tumor rises above the surface and grows into the dermis, survival rate decreases to 30–50%, since patients die from hematogenous metastases to the internal organs. The main factor in the prognosis of melanoma is the thickness of the tumor according to Breslow, which is measured in millimeters during histological examination.
“Thin” melanomas (Breslow tumor thickness 1 mm or less) have a favorable prognosis - 95% of five-year survival. With a tumor thickness of 2–4 mm, the prognosis sharply worsens, the five-year survival rate is 63–79%, and with a tumor thickness of 4 mm or more - 45% [2].
Melanoma can occur not only on unchanged skin, but also against the background of pre-existing formations: Dubreuil's melanosis or dysplastic nevus. According to the literature, 36% of sporadic and 70% of familial melanomas originate from dysplastic nevus.
Dysplastic nevus (Clark nevus, atypical nevus, lentiginous melanocytic dysplasia) was first described by WH Clark et al. in 1978 in two patients with hereditary nevi and melanoma [3]. The authors identified pigmented nevi, characterized by the proliferative activity of epidermal melanocytes with an increase in their atypia. The combination of multiple hereditary dysplastic nevi and melanoma in relatives is called “B-K-mol syndrome”, associated with a 90% risk of developing melanoma.
DE Elder et al. in 1980, described multiple sporadic (non-hereditary) nevi and found that in all cases of dysplastic nevi, the most common lesion is lentiginous melanocytic dysplasia (LMD), reminiscent of those melanocyte changes that occur in lentigo simplex [4].
Professor MNIOI named after. P. A. Herzen Z. V. Golbert in 1982 described three degrees of development of LMD, showing that the 3rd degree of LMD approaches the picture of melanoma in situ [5]. Currently, foreign authors distinguish between mild, moderate and severe cellular atypia: mild, moderate, severe [6]. LMD can occur alone (lentiginous dysplastic nevus) or be combined with nevoid structures in the dermis (mixed dysplastic nevus [3, 5, 7].
The cause of dysplastic nevi, which occur in 5-10% of the white population, is a mutation in the genetic apparatus, as well as exposure to external factors - solar radiation or ultraviolet irradiation in a solarium.
Dysplastic nevi differ from ordinary borderline nevi in the presence of proliferating melanocytes in the epidermis, as well as in the nature of development. The usual borderline nevus appears only in childhood and subsequently develops into a mixed nevus, and then into an intradermal nevus, which eventually loses pigment and becomes fibrotic [7].
Dysplastic nevi, appearing in adolescence, adulthood and old age, into old age, never turn into papillomatous intradermal nevi, i.e., the proliferative activity of melanocytes remains in them for a long time, which does not exclude possible transformation into melanoma. The fact that melanoma occurs extremely rarely in children appears to be associated with high antitumor immunity in childhood. As it decreases in adulthood and old age, the incidence of melanoma increases.
Clinically, dysplastic nevi are very similar to ordinary nevi and appear as pigmented spots or slightly raised formations. Unlike ordinary nevi, they are often irregular in shape and may have a “fried egg” appearance with a raised central part and a flat peripheral rim [7–9].
There are small nevi (up to 0.3 cm in diameter), medium (up to 0.7 cm) and large (more than 0.7 cm) [7]. The color of nevi can be heterogeneous, the color ranges from pink and reddish to dark brown and black, the edges can be smooth or wavy. Dysplastic nevi can be hereditary or sporadic.
Hereditary nevi, described by Clark in 1978, appear in childhood and adolescence; they are large, multiple, of various shades (from pink and reddish to brown and black), combined with papillomatous nevi, and are located in most cases on the body [3, 7].
Sporadic dysplastic nevi of brown color can be multiple or single, appear in adolescence and adulthood to old age, are small in size (0.1–0.5 cm), are often located on open areas of the body (outer surface of the upper extremities, upper half of the body ) [8–12]. The appearance of sporadic dysplastic nevi is promoted by frequent exposure to the sun or solarium.
Histologically, lentiginous dysplastic and mixed nevi are distinguished. In the first case, proliferating melanocytes are located in the epidermis at the border with the dermis, in the second, nevus cells are also found in the dermis. Clinically, these formations are not different: both are pigment spots or slightly raised formations. Pathomorphologist L.V. Chervonnaya believes that mixed dysplastic nevus, like a regular borderline nevus, is formed by the “accumulation” of proliferating melanocytes into the dermis, which indicates pronounced antitumor immunity [7].
Dysplastic nevi are benign formations. In most cases, they exist for a long time without any changes or regress. When such nevi are removed, grade 1–2 dysplasia (mild dysplasia) is histologically determined. All dysplastic nevi should not be removed. It is necessary to strive to remove dysplastic nevi with grade 2-3 dysplasia (severe dysplasia), which may be indicated by ABCDE signs: A (asymmetry) - irregular shape, B (border) - uneven, wavy edges, C (color) - uneven coloring, D (diameter) - more than 0.4 cm, E (evolving) - change in nevus over the past 10 years [10–13]. The main of the five listed signs is E, implying changes in a recently appeared or long-existing dysplastic nevus. Since 2020, nevi with grade 2–3 dysplasia are called progressive [11].
Materials and research methods
Since 2009, the surgical department of ZAO CP Litfond has been performing excisional biopsies of progressive dysplastic nevi with signs of ABCDE [10–13]. Histological examination is carried out at OJSC "Medicine", all melanoma preparations are sent for consultation to the Moscow Research Institute of Orthopedic Research. P. A. Herzen to his collaborator E. A. Yagubova.
It must be taken into account that not only progressive dysplastic nevus has clinical signs of ABCDE, but also early melanoma, however, unlike it, dysplastic nevus has a smooth surface and does not rise above the skin level. If the pigment formation was elevated and signs of ABCDE were present, we referred the patient with suspected melanoma to an oncology facility.
We use dermatoscopy as an auxiliary method to exclude non-melanocytic skin formations - keratomas, hemangiomas. It should be noted that in the presence of medium and large dysplastic nevi (0.4 cm in diameter or more), a progressive dysplastic nevus is detected upon examination with the naked eye and the use of dermatoscopy is not required. Its role increases with small nevi - 0.3 cm or less. In any case, the final diagnosis is established only after histological examination.
Excision of progressive dysplastic nevi was performed under local anesthesia, removing a flap with subcutaneous tissue to the fascia or to the muscle layer, with a distance of 0.5 cm from the visible boundaries, since such a distance is considered sufficient for melanoma in situ. In case of pronounced signs of ABCDE, which could indicate a “thin” melanoma, the boundaries were deviated by 1 cm, which made it possible to avoid re-operation when a “thin” invasive melanoma was detected.
Results and its discussion
In just 10 years (2009–2019), 174 lesions were excised in the surgical department of the Literary Fund Center with a clinical diagnosis of “progressive dysplastic nevus”. There were 146 women, 28 men, the age of the patients ranged from 22 to 77 years. The localization of pigment formations was different: head, neck, torso, upper and lower extremities. The size of the formations ranged from 0.25 to 1.8 cm in diameter.
Histological examination revealed early melanoma in 16 cases (9.2%), and dysplastic nevus in 133 cases (76.4%). In 27 cases (15.5%), grade 3 lentiginous melanocytic dysplasia was detected, that is, the diagnosis of “progressive dysplastic nevus” was confirmed. In 59 cases, degree 2 LMD was detected, in 34 - degree 1, in the rest - the degree was not established.
Of 133 dysplastic nevi, mixed dysplastic nevus was identified in 77 cases (57.9%), lentiginous nevus in 56 (42.1%). Melanoma in situ was detected in 6 cases (37.5%), invasive - in 10. The development of melanoma against the background of a dysplastic nevus was also noted in 6 of 16 cases (37.5%), de novo - in 10 cases (62.5% ).
The thickness of melanoma according to Breslow was 1 mm in 2 cases, in the rest - 0.75 mm, the level of invasion according to Clark was 2-3 with no ulceration, that is, in all cases, according to the WHO classification, melanoma was stage 1A, T1aN0M0 (2). The five-year survival rate at this stage is 95%. Our experience shows that it is not clinically possible to distinguish a progressive dysplastic nevus from melanoma in situ due to the similarity of symptoms; digital dermoscopic criteria have also not been developed. The diagnosis of melanoma in situ is currently established only by histological examination of the removed lesion.
Abroad, for the purpose of early diagnosis of melanoma, biopsy of dysplastic nevi is widely used, which in most cases is carried out by general practitioners and dermatologists, less often by surgeons and oncologists [9].
The large number of biopsies has led to a high detection rate of early cutaneous melanoma in the United States and Australia. In the United States, 48,290 in situ melanomas were reported in 2007. This means that 10 times more biopsies were performed (!) [2]. When performing a nevus biopsy in these countries, it is recommended to capture healthy tissue 0.2 cm wide. If melanoma is detected, reoperation is performed [9]. Some authors recommend reoperation even if moderate and severe cellular atypia (moderate-severe) is detected, if there is a positive resection margin, since it is often difficult to histologically draw the line between grade 3 LMD and melanoma in situ [14].
In the Russian Federation, very few biopsies of dysplastic nevi are performed compared to foreign countries, which explains the low detection rate of early melanomas. To increase the number of biopsies of dysplastic nevi, it is necessary to involve the entire army of doctors, as well as the nursing staff of primary care, that is, district clinics, in this problem. Physicians and dermatologists should identify dysplastic nevi, and surgeons should perform biopsies. Pathologists should also be required to submit information to the district oncology clinic when detecting melanoma in situ to take into account the 0th stage of melanoma - TisN0M0. It is by the number of melanomas in situ that one can judge how work on the early detection of skin melanoma is organized in a given state.
Based on our experience, it seems to us that the algorithm for early detection of melanoma in a district clinic should look like this.
- All outpatient doctors, as well as nursing staff, when examining a patient’s skin, should pay attention to unusual “moles” that have signs of ABCDE, as well as “new” moles that have appeared. Particular attention should be paid to the back surface of the torso, upper and lower extremities, that is, to those areas that are inaccessible for self-observation. Doctors and nursing staff of clinics should be provided with visual reminders depicting progressive dysplastic nevi and early melanoma.
- If a progressive dysplastic nevus is detected, the patient should be referred to a clinic surgeon to perform an excisional biopsy.
- An excisional biopsy should be performed by a clinic surgeon under local anesthesia; it is necessary to excise a flap of skin with subcutaneous tissue down to the fascia with a distance of 0.5 cm from the visible boundaries for mildly expressed ABCDE signs, and 1.0 cm for pronounced signs. Excisional biopsy of a pigmented formation should not be performed , if it rises by more than 0.1 cm, in these cases the patient should be referred to an oncologist with suspected melanoma.
- Histological examination of removed dysplastic nevi should be carried out by a pathologist with experience in studying melanocytic formations, since it is difficult to distinguish melanoma in situ from a progressive dysplastic nevus (lentiginous melanocytic dysplasia grades 1–3).
- If invasive melanoma is detected by histological examination, the patient should be referred to an oncologist at his place of residence for registration and regular examination.
The Russian Federation has a well-developed network of clinics with surgical offices, so there should be no obstacles to excisional biopsy of progressive dysplastic nevi. This operation should become routine in the practice of an outpatient surgeon. Our ten-year experience in excision of dysplastic nevi in the surgical department of the Literary Fund Center, as well as foreign experience, shows: the more biopsies of dysplastic nevi are performed, the more early melanomas are detected, which ultimately leads to a decrease in mortality from this cancer.
Here are extracts from medical records
Clinical case No. 1
Patient L., 39 years old, consulted an oncologist on October 13, 2014 about a “mole” in the lumbar region on the left, which appeared about a year ago. On examination: in the lumbar region on the left there is a nevus 1.0 × 0.5 cm, dark brown in color, irregular in shape, with wavy edges (Fig. 1). Diagnosis of “progressive dysplastic nevus”. The nevus was excised on October 13, 2014 under local anesthesia, at a distance of 1.0 cm from the visible boundaries. Histological examination revealed a mixed pigmented nevus with grade 2–3 LMD.
Clinical case No. 2
Patient V., 59 years old, consulted an oncologist about skin formations in the lumbar region on October 16, 2015. On examination, in the lumbar region on the left there is a dysplastic nevus, dark brown in the center and lighter along the periphery, measuring 1.4 × 0.4 cm (Fig. 2). Excision of the nevus with histological examination is recommended. The patient refused the operation and came back a year later because she injured the mole with a nail. Upon examination, no significant dynamics of the nevus were noted; there were bloody crusts on the surface. The diagnosis is a traumatized pigmented nevus. On September 21, 2016, the nevus was excised under local anesthesia. Histological examination - lentigo melanoma, level 2 invasion according to Clark, thickness according to Breslow - less than 0.75 mm.
Clinical case No. 3
Patient Zh., 29 years old, consulted an oncologist on October 28, 2016 about a “mole” on her right leg, which appeared a year ago. From the anamnesis: my grandmother has skin melanoma. On examination: in the lower third of the right leg on the outer surface there is a nevus 0.4 × 0.4 cm, irregular in shape, heterogeneous color: dark brown in the center, light brown along the periphery (Fig. 3). Diagnosis of “progressive dysplastic nevus”. The formation was excised on November 10, 2016 under local anesthesia, 1 cm away from the visible boundaries. Histological examination: mixed dysplastic nevus with lentiginous melanocytic dysplasia of the 3rd degree.
Clinical case No. 4
Patient Z., 57 years old, was referred by a dermatologist regarding a “mole” on his thigh. Upon examination on April 27, 2018: on the torso and limbs there were multiple freckles and spots like Dubreuil’s melanosis. In the middle third of the posterior surface of the left thigh there is a pigment spot 0.6 × 0.6 cm, irregular in shape, with uneven edges, and heterogeneous color (Fig. 4). According to the patient, the “mole” on the thigh lasts no more than 5 years. The diagnosis is progressive dysplastic nevus. On October 23, 2018, the formation was excised under local anesthesia, at a distance of 1 cm from the visible boundaries. Histological examination revealed epithelial cell non-ulcerated melanoma, in situ, partially lentigo-, partially superficially spreading, with tumor cells infiltrating the entire thickness of the squamous epithelial cover. Subepithelial - pronounced lymphoid-plasma cell infiltration.
Clinical case No. 5
Patient K., 52 years old, consulted a dermatologist on May 20, 2019 about a “mole” on her left forearm that appeared in April 2020, and was referred to an oncologist. She contacted the oncologist a month later, on 07/09/19. Upon examination, in the middle third of the left forearm on the dorsal surface there is a slightly raised formation 0.4 cm in diameter, round in shape, light brown in color, with a dark area in the center (Fig. 5 ). The axillary lymph nodes are not enlarged. Diagnosis of “progressive dysplastic nevus”. The formation was excised on July 16, 2019 under local anesthesia, at a distance of 1 cm from the visible boundaries. Histological examination - superficial spreading melanoma, Breslow thickness - 1 mm, 3rd level of invasion according to Clark, with mild lymphoid infiltration, without signs of vascular and perineural invasion.
We express our gratitude to the pathologist of the Moscow Research Institute named after. P. A. Herzen, Ph.D. Emma Aleksandrovna Yagubova, official consultant of the pathomorphological department of OJSC "Medicine", for consultations on preparations of dysplastic nevi and melanomas of the skin.
Literature
- Kaprin A. D., Starinsky V. V., Petrova G. V. Malignant neoplasms in Russia in 2020 (morbidity and mortality). M., 2020.
- Usatine R. P. Melanoma. Atlas-reference book for a practicing physician / Transl. from English 2012. pp. 324–333.
- Clark WH, Reimer RR, Greene M, Ainsworth AM, Mastrangelo MJ Origin of Familial Malignant Melanomas from Heritable Melanocytic Lesions. The BK-mole Syndrom // Archives of Dermatology. 1978. Vol. 114, no. 5, 732–739.
- Elder DE, Leonardi J, Goldman J, Goldman SC, Greene MH, Clark WH Displastic Nevus Syndrome. A Pfenotypic Association of Sporadic Cutaneous Melanoma // Cancer. 1980, no. 8.
- Golbert Z.V., Chervonnaya L.V., Klepikov V.A., Romanova O.A. Lentiginous melanocytic dysplasia as a precursor to the development of malignant melanoma // Archives of Pathology. 1982; 12:36–41.
- Hale CS Dysplastic nevus. 1 September 2014, revised 24 May 2019. Copyright 2003–2019. Pathology Outlines.com, inc.
- Chervonnaya L.V. Pigmented tumors of the skin. M.: IG GEOTAR-Media, 2016. pp. 71–86.
- Romanova O. A. Early diagnosis and prevention of skin melanoma. Atlas guide. M.: MIA, 2012. pp. 22–25.
- Smith M. A. Dysplastic nevus. Dermatology. Atlas-reference book for a practicing physician / Transl. from English 2012. pp. 288–291.
- Romanova O. A., Artemyeva N. G. Surgical prevention of skin melanoma // Oncosurgery. 2013; 3:12–18.
- Romanova O. A., Artemyeva N. G., Yagubova E. A., Rudakova I. M., Marycheva V. N., Veshchevailov E. A. Tactics for managing a patient with a dysplastic nevus // Clinical dermatology and venereology. 2015; 2:92–97.
- Romanova O. A., Artemyeva N. G., Yagubova E. A., Rudakova I. M., Marycheva V. N., Veshchevailov E. A. Principles of excisional biopsy of a dysplastic nevus in an outpatient setting // Oncology. 2016; 1:36–41.
- Romanova O. A., Artemyeva N. G., Solokhina M. G. Signs of ABCDE in the diagnosis of dysplastic nevus with signs of progression and initial melanoma // Treating Physician. 2016; 9:92–95.
- Reddy KK, Farber MJ, Bhavan J., Geronemus RG, Rogers GS Atypical (dysplastic) nevi outcomes of surgical excision and association with melanoma // JAMA Dermatol. 2013, Aug; 149(8):928–34. DOI: 10.1001/jamadermatol.2013.4440.
O. A. Romanova*, 1, Candidate of Medical Sciences N. G. Artemyeva*, Candidate of Medical Sciences V. N. Marycheva** L. R. Kurashvili**, Candidate of Medical Sciences
* JSC "CP Literary Fund", Moscow ** JSC "Medicine", Moscow
1 Contact information
DOI: 10.26295/OS.2020.19.87.004
Algorithm for early detection of skin melanoma in a district clinic / O. A. Romanova, N. G. Artemyeva, V. N. Marycheva, L. R. Kurashvili For citation: Attending physician No. 5/2020; Page numbers in the issue: 22-26 Tags: skin, malignant tumors, metastases, diagnostics
Survival prognosis
Several reasons influence the survival prognosis. Women tolerate the disease better than men, since they most often have melanomas on the extremities, and this is a more positive sign. It is worst if the disease affects the neck, back of the head or upper back. Thickness, changes in contours and pigmentation of the tumor also play a role. If melanoma grows vertically, then this is also a more unfavorable sign than the horizontal direction.
Melanoma affects people regardless of age: from children to the elderly. Therefore, it is necessary to prevent the development of the malignant process until the last stage; it is better to eliminate it at the very beginning, when there are no metastases yet.
Classification according to criterion M (presence of distant metastases)
As we have already said, all metastases that extend beyond the zone of regional lymph nodes or affect internal organs are distant. Here are the following options:
- M0 - no distant metastases.
- M1 - there are distant metastases. In this case, this stage is divided into the following substages: M1a - there is metastatic damage to the skin, subcutaneous tissue and lymph nodes (not regional) with a normal level of lactate dehydrogenase (LDH).
- M1b - melanoma metastases affect the lungs, LDH levels are normal.
- M1c - there are metastases to other internal organs, or metastases to the skin, subcutaneous tissue or lymph nodes with elevated LDH levels.
Clinical manifestations
It is very important to correctly diagnose this dangerous disease. For example, it can be confused with seborrheic dermatitis, warts vulgaris and basal cell carcinoma. Clinical signs of stage 3 melanoma:
- constant severe headaches that do not respond to painkillers;
- melanosis - an excess amount of pigment, melanin, in the body;
- compaction of the neoplasm with the appearance of cracks;
- the occurrence of pain when touching a mole;
- rapid weight loss for no apparent reason;
- enlargement of lymph nodes located close to the site of the disease.
Classification of melanoma according to T criterion
pTx - there is insufficient data for histological assessment of the thickness of melanoma. Such situations arise when melanoma undergoes spontaneous regression, or when the tumor is damaged during surgical excision.
pT0 - no primary tumor.
PTis is melanoma in situ, which corresponds to level 1 of invasion according to Clark, when malignant melanoma cells are located only in the epidermal layer. Clinically, the diagnosis may sound like melanocytic dysplasia.
pT1 - melanoma with the first stage of invasion according to Breslow, i.e. less than 1 mm. This stage is divided into 2 substages:
- pT1a - the tumor affects the papillary layer of the dermis (including its entire thickness), but there are no ulcerations on its surface.
- PT1b - the tumor extends beyond the papillary layer and extends to the reticular layer of the dermis or subcutaneous tissue, or the tumor does not extend beyond the papillary layer, but there are ulcerations on its surface.
pT2 - melanoma with a Breslow degree of invasion within 1-2 mm. Divided into 2 stages:
- pT2a - there are no ulcerations on the surface of the melanoma.
- PT2b - there are ulcerations on the surface of the melanoma.
pT3 - melanoma with a Breslow degree of invasion within 2-4 mm. Divided into 2 stages:
- pT3a - there are no ulcerations on the surface of the melanoma.
- PT3b - there are ulcerations on the surface of the melanoma.
pT4 - melanoma with a Breslow degree of invasion of more than 4 mm. Divided into 2 substages:
- pT4a - there are no ulcerations on the surface of the melanoma.
- PT4b - there are ulcerations on the surface of the melanoma.
Diagnosis and treatment of relapses
Stage three melanoma is characterized by unpredictability and high aggressiveness. Therefore, even after complete removal of malignant tumors, constant regular examination of the patient is necessary. In the first two years, routine monitoring is recommended at least once every three months. In the next three years - at least once every six months and then annually. Unfortunately, it is impossible to guarantee a complete cure for the disease and the absence of relapses in the future, since even one cancer cell remaining in the lymph channel or vessel can trigger the return of the disease. However, it is impossible to predict the complexity of a recurrent case, which may be of the same volume as the primary melanoma or greater.
Surgical method of treatment
Melanoma is a very aggressive cancer characterized by rapid growth and spread throughout the body. Therefore, when detected in a patient, treatment must be started immediately. At the third stage, the process includes mandatory surgical intervention, which includes removal of the source of the disease and affected tissue, as well as excision of nearby healthy tissue. The thicker the melanoma, the greater the volume of healthy skin that must be captured during removal. In some cases, it becomes necessary to transplant skin to the affected areas.